Corn Field IMage Petr Kratochvil.jpg
Forbes
on August 24, 2022)
(Cornfield Image – Petr Kratochvil)
Critics of modern agriculture often cite its
dependence on “synthetic nitrogen fertilizers.” They point to the carbon
footprint of the natural gas used to make it and the fact that nitrogen from
farms can end up as a water or air pollutant. Also there is the issue that under
certain circumstances a fraction of farm applied nitrogen can be emitted from
the soil as the very potent greenhouse gas – nitrous oxide. While these issues
are real, the solution is not to somehow avoid using this fertilizer or to
arbitrarily set limitations on the quantities that farmers can use to grow their
crops. Unfortunately there are several such misguided approaches being pursued.
A cautionary tale comes from the nation of Sri Lanka which recently banned
fertilizer imports in its attempt to become the first 100% organic production
region. That choice crippled their food supply and their important tea export
industry. Canada recently mandated nitrogen fertilizer reduction for its farmers
without tying that to a rational measure such as kg of fertilizer per ton of
output. India is promoting “zero-budget natural farming which could undermine
the food independence that nation has enjoyed since the “green revolution.”
The European Union is promoting organic agriculture which excludes “synthetic
fertilizers” as part of its controversial Farm to Fork Strategy. In fact,
organic is often promoted as a solution to fertilizer issues without recognition
that organic crop production is actually quite dependent on “synthetic” nitrogen
that has ended up in the manure of conventionally raised animals. As one expert
on the subject says, “follow the nitrogen.”
And just to be clear, human produced nitrogen fertilizer starts as ammonia which
is a naturally occurring form of that element and not something artificial as the
term “synthetic” might imply.
Getting Fertilization “Just Right” In the classic fairy tale Goldilocks wants
her porridge “not too hot, not too cold, but just right.” The challenge for
farmers is to apply nitrogen in a way that doesn’t represent either too much or
too little, but what is “just right” for optimal crop growth. Fertilizers are
one of the more significant operating costs of growing a crop, so growers have
no incentive to over-apply. But conversely if a crop is short on nutrients
during key growth stages, the farmer’s yield-based income will be compromised.
Thus, the long-standing goal for optimal fertilization has been expressed as
“The 4-Rs” –
1. the right amount
2. in the right form
3. in the right place
4. at the right time
This is a non-trivial challenge because of logistical
limitations and the vagaries of weather, but the basic economics drive careful
use. Why Agriculture Needs to “up it’s N-game” There are now two “game changing”
factors driving more attention to nitrogen fertilizer issues -the war in Ukraine
and Climate Change. The war has led to a dramatic increase in fertilizer prices
and highlighted the desirability of a to shift to domestic sourcing. Rational
concern about Climate Change is putting the spotlight on the greenhouse gas
footprint of current nitrogen fertilizer production methods as well as on the
emissions of nitrous oxide from fields.
In the face of these heightened concerns
the agricultural sector is being called upon to “up its N-game.”
Why Agriculture Needs to Up it’s “N-Game”
The challenge is to meet increasing demand for food, feed, fiber, fuel and other biomaterials
without driving land-use-change and without exacerbating nitrogen-related
issues. Fortunately, the trend over the past three decades is encouraging.
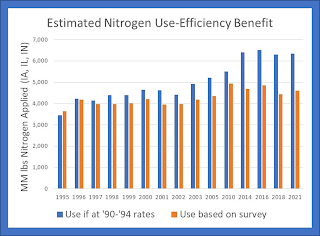
Consider the example the “I states” which account for around one third of US
grain corn production. As shown in the graphs below, yield in 2021 was 35-51%
higher than in the early 1990s but nitrogen use only increased between 8 and
18%. Thus “nitrogen use-efficiency” in those states (expressed as bushels
produced per pound of nitrogen applied) has increased 29-35%.
That means that the use of nitrogen for corn in these three states in 2021 was
1.73 billion pounds lower than if it has been used at the rates it was in the
early 1990s. That benefit is shown below for each year in which there was survey
data.
Farmers use many different practices and technologies in order to optimize their
use of nitrogen and other fertilizers. The following is a list of both existing
and emerging n-game tactics. Some are well established but could be more widely
employed. Those are highlighted with the symbol (>>>). Others that are
relatively new, but which could make a significant contribution are highlighted
with the symbol (+++). Those that are in the research phase are indicated by the
symbol (***). Nutrient Recovery When animals (including humans) digest their
food they fail to absorb all the nutrients it contains. That is why manure has
always been used as a fertilizer as it continues to be today. Manure in its
various forms (including after composting) is not an ideal fertilizer in that it
requires the application of tons per acre and it isn’t amenable to some
desirable farming practices such as no-till farming or precision application
(described below). Even so, a new technology called a Varcor Processor (+++) is
available today to do a much better job of recovering the fertilizer nutrients
from manure in highly usable forms. There is also interest in setting up
mechanisms to recycle human urine (***) as a fertilizer high in both nitrogen
and phosphorus. However, since neither animals or humans actually make nitrogen
fertilizer, these are limited potential sources. Precision Fertilization Farm
field soils are not uniform in that they have different yield potential in
different zones. It is common today for farm machinery to be equipped with GPS
or other geo-referencing technologies in order to do “auto-steer” and to
generate information like a yield map. To avoid wasting money on excess
fertilizer the farmer can use “variable rate fertilization” (>>>) putting down
more or less in each individual zone based on its growth potential. The
application rates can also be guided by various imaging technologies that use
“hyperspectral analysis” (>>>) to visualize the nutrient status of the growing
crop and to adjust fertilizer rates on that even more precise zone basis. For
crops that are irrigated it is possible to very closely link the supply of
nitrogen and other nutrients to what the plants need at any given point in the
growing season by “spoon feeding” (>>>)- delivering it through drip lines or
other irrigation systems at levels that closely match what the plants will
quickly absorb with their roots at each time point throughout the season. In
non-irrigated agriculture that level of control is not possible, but fertilizer
can be applied in a few “split applications” (>>>) to more closely match plant
needs. Another option is a “controlled release formulation” (>>>) of the
fertilizer in which a polymer coating slows the rate at which the nutrients move
out into the soil. Preventing Nitrogen Loss After a nitrogen fertilizer has been
applied in a field it can be a while before it is taken up by the growing crop
and in the meantime, it can be converted to forms that allow it to move into the
air or water so that it is no longer available for the crop and can cause
problems in the environment. There are several technologies that act as
“Nitrogen Loss Inhibitors.” For instance urea is a very practical form of
nitrogen to use as a fertilizer, but there are enzymes present in soils called
ureases that convert it to ammonia (NH4) which is volatile so that it moves away
in the atmosphere only to be washed down later and cause a form of water
pollution known as “eutrophication.” There are products called “urease
inhibitors” (>>>) that prevent that potentially major form of nitrogen loss.
When fertilizer nitrogen is in the positively charged ammonium form (NH4+) it is
in an available but non-mobile form. There are microbes in the soils that
convert the ammonium to nitrate (NO3-) which is very mobile in water so that it
can leach into ground water or be washed into streams. If the soil is
waterlogged or compacted so that it doesn’t have air available, the nitrate can
also be lost to the crop if it is “denitrified” meaning that it is converted to
N2 gas that goes back into the air as a harmless gas. Unfortunately, in that
process some of the nitrogen is turned into nitrous oxide (N2O) which is an
extremely potent greenhouse gas. There are products called “nitrifications
inhibitors” (>>>) which reduce these nitrogen loss and pollution issues. The
GPS-based autosteer technology also allows the grower to employ “controlled
wheel trafficking” (>>>) so that only a small percent of the field is ever
compacted by the wheels of heavy equipment. If no nitrogen fertilizer is applied
to those potential soil compaction wheel tracks, the risk of nitrous oxide
emissions is greatly diminished. Using “Green” Nitrogen In the early
20thcentury, the German scientists Fritz Haber and Karl Bosch invented the
process through which the inert nitrogen gas that makes up 78% of the atmosphere
could be converted into ammonia and from the converted to other forms that can
fertilize plants. Up until that time the world had been tapped out of nitrogen
from natural sources including the mining of deposits of bird guano. Hydrogen is
also needed for that reaction and natural gas (CH4) has has always been used in
the Haber-Bosch process because it was the cheapest source. Hydrogen can also be
produced from water using wind or solar generated electricity and there are
technologies now available to make much lower carbon footprint nitrogen (+++)
and they are getting more cost competitive. Another advantage of using renewable
energy to generate nitrogen fertilizer is that it can help with the reduction of
import dependence. Nitrogen Fixers The term “fixer” has some negative
connotations, but in nature there are certain beneficial bacteria that can “fix”
nitrogen meaning they have a unique ability to take some of the nearly inert N2
gas that makes up 78% of the atmosphere and convert it into ammonia (NH4) which
is the starting point for all the biologically important forms of that element.
There is a family of plants known as legumes that have a special relationship
with one of these bacterial species called Rhizobium. The plant supplies the
microbe with the sugars that then provide the considerable amount of energy
required for that process. The plants also “house” these bacteria in specialized
structures along their roots called “nodules” that created a very low oxygen
environment which is also important for the fixing process. Several major and
minor legume crops have this capability and require little to no nitrogen
fertilizer (soybeans, dry edible beans, peas, peanuts, lentils, chickpeas,
alfalfa…). When legumes are part of the crop rotation (>>>) they leave a fair
amount of nitrogen for the next non-legume crop (e.g. corn, wheat, canola…).
There are also legumes that can be used as cover crops (>>>) between seasons to
increase the supply of nitrogen in the soil.
Source link
2022-10-30 17:11:00
Karl Hoffman is a distinguished agriculturalist with over four decades of experience in sustainable farming practices. He holds a Ph.D. in Agronomy from Cornell University and has made significant contributions as a professor at Iowa State University. Hoffman’s groundbreaking research on integrated pest management and soil health has revolutionized modern agriculture. As a respected farm journalist, his column “Field Notes with Karl Hoffman” and his blog “The Modern Farmer” provide insightful, practical advice to a global audience. Hoffman’s work with the USDA and the United Nations FAO has enhanced food security worldwide. His awards include the USDA’s Distinguished Service Award and the World Food Prize, reflecting his profound impact on agriculture and sustainability.